复宏汉霖创新产品抗GARP/TGF-β1单抗获国家药监局临床试验批准

内容来源:复宏汉霖
2024年3月12日,复宏汉霖(2696.HK)宣布,公司自主开发的创新型抗GARP/TGF-β1单抗HLX6018的临床试验申请已经获得国家药品监督管理局批准,用于特发性肺纤维化(idiopathic pulmonary fibrosis,IPF)的治疗。HLX6018为复宏汉霖在慢性炎症性疾病领域布局的首款创新产品。目前,全球范围内尚无同靶点产品获批上市。

纤维化是一种以组织瘢痕为特征的病理过程,多因感染、自身免疫反应、辐射、机械损伤等因素引发细胞外基质(extracellular matrix, ECM)过度沉积,导致正常组织变成永久性组织瘢痕,常见的纤维化相关疾病包括IPF、非酒精性脂肪性肝炎/代谢功能障碍相关脂肪性肝炎、肝硬化、慢性肾脏疾病、心肌梗死等[1]。其中, IPF是一类发病机制尚不明确的慢性进行性间质性肺病,于2018年被列入国家《第一批罕见病名录》-1。据统计,全球范围内已有约200万IPF患者,且发病率和死亡率仍在不断提升-1。该疾病多发于中老年男性,患者因肺部的进展性纤维化造成肺功能不可逆转地丧失,最终导致死亡。且IPF预后差,患者诊断后中位生存期仅为3-5年,五年生存率不足30%,甚至低于多类肿瘤疾病[2]。目前,IPF的治疗选择非常有限,且现存药物仅能延缓肺功能下降,安全、有效的抗肺纤维化治疗药物存在很大的未满足的临床需求。
转化生长因子-β(transforming growth factor-β, TGF-β)是一种多效细胞因子,在所有类型的组织纤维化发生和发展过程中都发挥着关键作用。研究表明,TGF-β1通过激活Smad依赖性或非依赖性信号通路,介导肌纤维母细胞(Myofibroblast)的激活、ECM的过量产生和ECM降解的抑制等,从而引起肺、肝、肾和心脏等器官的纤维化-2。糖蛋白A重复优势蛋白(glycoprotein-A repetitions predominant,GARP)是潜在转化生长因子β1(latent transforming growth factor-β1, LTGF-β1)的对接受体,表达于血小板及多类细胞表面。GARP通过与LTGF-β1结合形成GARP-LTGF-β1复合物,进而促进成熟TGF-β1的分泌和活化,成为TGF-β1成熟释放的重要途径之一-1。
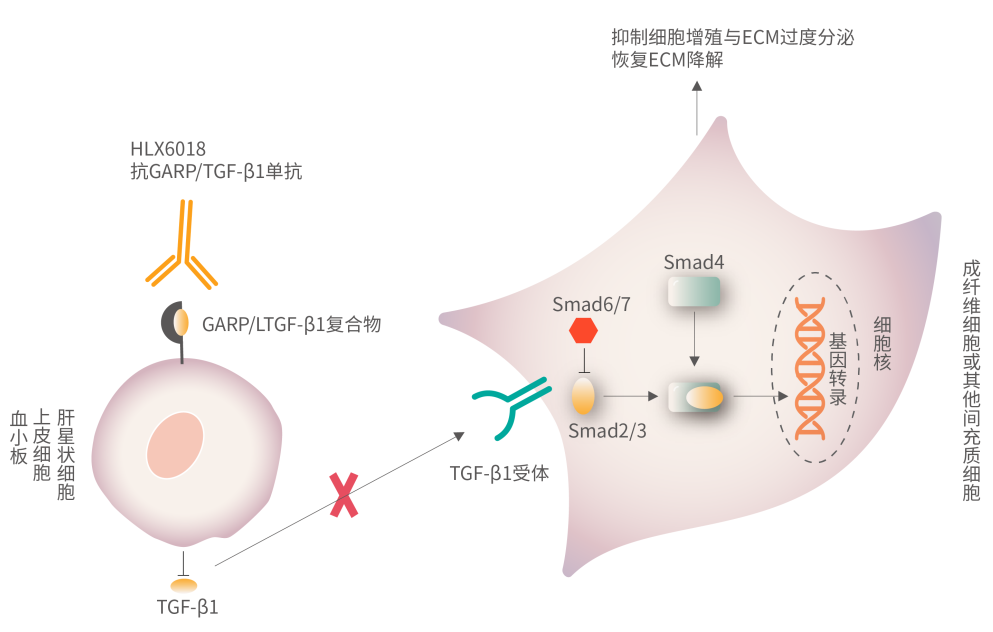
HLX6018能够特异性结合细胞及血小板表面的GARP/TGF-β1复合物,从而阻断GARP介导的TGF-β1成熟释放,进而抑制TGF-β1介导的成纤维细胞的激活、增殖和ECM的分泌,达到治疗纤维化相关疾病的目的。临床前研究中,HLX6018已经展现出显著的抗肺纤维化及肾纤维化作用,且具有良好的安全性。除特发性肺纤维化外,后续,复宏汉霖也将继续探索HLX6018在肾纤维化、肝纤维化等更多纤维化疾病中的疗效。
聚焦未满足的临床需求,复宏汉霖将持续加码创新研发,以肿瘤为基石,不断拓展非肿瘤领域的治疗方案,加速开发更多安全有效的创新药物,以可负担的高品质创新药惠及全球患者。
关于复宏汉霖
复宏汉霖(2696.HK)是一家国际化的创新生物制药公司,致力于为全球患者提供可负担的高品质生物药,产品覆盖肿瘤、自身免疫疾病、眼科疾病等领域,已在中国上市5款产品,在国际上市2款产品,19项适应症获批,7个上市申请分别获中国药监局、美国FDA和欧盟EMA受理。自2010年成立以来,复宏汉霖已建成一体化生物制药平台,高效及创新的自主核心能力贯穿研发、生产及商业运营全产业链。公司已建立完善高效的全球创新中心,按照国际药品生产质量管理规范(GMP)标准进行生产和质量管控,不断夯实一体化综合生产平台,其中,上海徐汇基地和松江基地(一)均已获得中国和欧盟GMP认证。
复宏汉霖前瞻性布局了一个多元化、高质量的产品管线,涵盖20多种创新单克隆抗体,并全面推进基于自有抗PD-1单抗H药汉斯状®的肿瘤免疫联合疗法。继国内首个生物类似药汉利康®(利妥昔单抗)、中国首个自主研发的中欧双批单抗药物汉曲优®(曲妥珠单抗,欧洲商品名:Zercepac®,澳大利亚商品名:Tuzucip®和Trastucip®)、汉达远®(阿达木单抗)和汉贝泰®(贝伐珠单抗)相继获批上市,创新产品汉斯状®(斯鲁利单抗)已获批用于治疗微卫星高度不稳定(MSI-H)实体瘤、鳞状非小细胞肺癌、广泛期小细胞肺癌和食管鳞状细胞癌,并成为全球首个获批一线治疗小细胞肺癌的抗PD-1单抗。公司亦同步就16个产品在全球范围内开展30多项临床试验,对外授权全面覆盖欧美主流生物药市场和众多新兴市场。
The IND Application of Henlius’ Novel Anti-GARP/TGF-β1 mAb HLX6018 Approved by NMPA
Shanghai, China, Mar 12th, 2024 - Shanghai Henlius Biotech, Inc. (2696.HK) announced the investigational new drug (IND) application for clinical trial of HLX6018,a novel anti-GARP/TGF-β1 monoclonal antibody (mAb) independently developed by the company, was approved by the National Medical Products Administration (NMPA) for the treatment of idiopathic pulmonary fibrosis (IPF). HLX6018 is the first innovative product of Henlius in the treatment field of chronic inflammatory diseases. Currently, no mAb targeting GARP/TGF-β1 has been approved for marketing globally.
Fibrosis is a pathological process characterised by persistent tissue scars which attributed to excessive deposition of extracellular matrix (ECM). This condition can be induced by a variety of stimuli such as infections, autoimmune reactions, radiation, and tissue injury. Common fibrosis-related diseases include IPF, non-alcoholic steatohepatitis (NASH)/metabolic dysfunction-associated steatohepatitis (MASH), cirrhosis, chronic kidney disease (CKD), myocardial infarction (MI), etc[1]. Among them, IPF is a chronic, progressive interstitial lung disease with unknown etiology, and was incorporated into the first National List of Rare Diseases in 2018-1. It is estimated that there are almost 2 million IPF patients in the globe, with its incidence and mortality still rising-1. IPF occurs mostly in elderly men, and the patients suffer from irreversible loss of lung function due to progressive lung fibrosis, which ultimately leads to death. Furthermore, the overall prognosis of IPF is poor, with the median survival of patients is only 3-5 years after diagnosis, and five-year survival rate less than 30%, which is even lower than multiple types of tumour[2]. Currently, treatment options for IPF are very limited, and have just shown their potential for delaying the decline of lung function. There is a large unmet clinical need in the treatment of IPF.
Transforming growth factor-β (TGF-β) is a pleiotropic cytokine and plays critical roles in the initiation and progression of all types of tissue fibrosis. Recent evidence shows that TGF-β1 can induce fibrosis via activation of both Smad-based and non-Smad-based signaling pathways, which result in activation of myofibroblasts, excessive production of ECM and inhibition of ECM degradation-2. Glycoprotein-A repetitions predominant (GARP) is highly expressed on the surface of platelets and other cell types and acts as a docking receptor by binding to latent transforming growth factor-β1 (LTGF-β1). Subsequently, mature TGF-β1 was further activated and released from the GARP/LTGF-β1 complex-1.
HLX6018 is Henlius’ self-developed novel anti-GARP/TGF-β1 mAb that binds to GARP/TGF-β1 complex and specifically blocks the release of GARP mediated TGF-β1, thus repressing the fibroblasts proliferation and ECM secretion caused by TGF-β1, and alleviating fibrosis symptoms. In preclinical studies, HLX6018 has demonstrated antifibrotic efficacy in both pulmonary and renal fibrosis models, and has a favorable safety profile. Afterwards, Henlius will continue to explore the efficacy of HLX6018 in a variety of fibrotic diseases including kidney fibrosis and liver fibrosis, steadily enrich the product’s indication layout in antifibrotic area.
Focusing on unmet medical needs, Henlius will further take efforts to promote the layout of its innovative portfolio, progressively expand the company’s therapeutics in non-oncology areas while taking oncology as a cornerstone, benefiting global patients with affordable, high-quality innovative medicines.
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable, and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases, and ophthalmic diseases. Up to date, 5 products have been launched in China, 2 has been approved for marketing in overseas markets, 19 indications are approved worldwide, and 7 marketing applications have been accepted for review in China, the U.S., and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation center and Shanghai-based manufacturing facilities in line with global Good Manufacturing Practice (GMP), including Xuhui Facility and Songjiang First Plant, both certificated by China and the EU GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering over 20 innovative monoclonal antibodies (mAbs) and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as backbone. Apart from the launched products HANLIKANG (rituximab), the first China-developed biosimilar, HANQUYOU (trastuzumab for injection, trade name in Europe: Zercepac®; trade names in Australia: Tuzucip® and Trastucip®), the first China-developed mAb biosimilar approved both in China and Europe, HANDAYUAN (adalimumab) and HANBEITAI (bevacizumab), the innovative product HANSIZHUANG has been approved by the NMPA for the treatment of MSI-H solid tumors, squamous non-small cell lung cancer (sqNSCLC) and extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC), making it the world's first anti-PD-1 mAb for the first-line treatment of SCLC. What's more, Henlius has conducted over 30 clinical studies for 16 products, expanding its presence in major markets as well as emerging markets.
【参考文献】
[1] Wynn T A . Cellular and molecular mechanisms of fibrosis.Journal of Pathology, 2010, 214(2):199-210.
[2] Wu W, et al. BMJ Open 2021; Effcacy and safety of pirfenidone in the treatment of idiopathic pulmonary fbrosis patients: a systematic review and meta-analysis of randomized controlled trials. 11:e050004.
[3] 国家卫生健康委员会等《第一批罕见病名录》
[4] Maher, T.M., Bendstrup, E., Dron, L. et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res 22, 197 (2021).
[5] 《中国特发性肺纤维化患者行业发展现状研究与投资前景预测报告》
[6] Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325-38.
[7] Ghafoory S, Varshney R, Robison T, et al. Platelet TGF-β1 deficiency decreases liver fibrosis in a mouse model of liver injury. Blood Adv. 2018 Mar 13;2(5):470-480.
[8] Inui N, Sakai S, Kitagawa M. Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway. Int J Mol Sci. 2021 Jun 5;22(11):6107.
[9] Tran DQ, Andersson J, etc. GARP(LRRC32)is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106(32) :13445-13450.
[10] Metelli A, Wu BX, Riesenberg B, et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-β. Sci Transl Med. 2020;12(525): eaay4860.